WOMEN’S WEIGHTLOSS FORMULA PROBIOTIC RESEARCH ON NEW BACTERIA STRAINS
Following are the benefits and the research notes conducted on our powerful ingredients.
LACTOBACILLUS PLANTARUM
- Associated with weight loss 1
- Reduced growth and penetration of pathogens.
- Prevents pathogens from penetration and entrance through the gut’s protecting layers into the body. 2
- Stimulate the secretion of mucin, which hinders the growth of disease-promoting bacteria. 3
- secreting antitoxins that seem to slow down pathogen expansion , such as hydrogen peroxide and benzoic acid. 4
- Produces SCFAs lowering colon luminal pH, creating an unfavorable environment for the growth of pathogens including molds, yeasts, and bacteria. 5
- Reduces circulating endotoxins (LPS), which would otherwise cause over-stimulation of the immune system and associated infections and inflammation. 6
- Produces antifungal compounds that has antifungal activities. 7
- Binding of Ochratoxin A11
- One of the most abundant food-contaminating mycotoxins. 12
- Human exposure can occur through consumption of contaminated food products, particularly contaminated grain and pork products, as well as coffee, wine grapes and dried grapes 13 14 15
- Ochratoxin A is potentially carcinogenic to humans possibly by induction of oxidative DNA damage 16
- Neurotoxicity- Ochratoxin A has a strong affinity for the brain, especially the cerebellum (Purkinje cells), ventral mesencephalon and hippocampal structures. 17 The affinity for the hippocampus could be relevant to the pathogenesis of Alzheimer’s disease
- Ochratoxin causes acute depletion of striatal dopamine, which constitutes the bed of Parkinson’s disease, however it did not cause cell death in any of brain regions examined. 18
- The developing brain is very susceptible to Ochratoxin, hence the need for caution during pregnancy. 19
LACTOBACILLUS RHAMNOSUS
- Summary: Facilitates weight loss, Resistant to gastric acid and bile, Strongly adheres to intestinal epithelial cells which may be important for competition with pathogens, stimulation of mucous production and the modulation of the host immune system. Maintains the intestinal barrier, inhibits intestinal pathogens and competes with them. Beneficial modulation of the immune system. Strongly down regulates the inflammatory responses
- After the 12-week weight-loss period, the women in Group #1 (probiotic group) lost almost 10 pounds, on average, while the women in Group #2 (placebo) lost only about 6 pounds. And then During the maintenance period, the women in Group #2 maintained a stable weight. Yet the women in Group #1 continued to slim down, losing almost 2 more pounds, on average. 20
- Produces antifungal metabolites 21
- Bind up Aflatoxins in vitro and effective at removing aflatoxin B1. 22. Aflatoxin- common in nuts, corn, spices, copra (dried coconut), and figs. They are known to be human carcinogens. They readily bind up macromolecules including DNA and proteins’ forming adducts which lead to cancer. 23
- 24 T-2 toxin and DON (deoxynivalenol) toxins frequently present in our daily intake of grains have been known to induce the growth of aerobic intestinal bacteria in pigs. 25 DON and T-2 toxin are the only identified mycotoxins capable of altering the microbial balance of the intestine (dysbiosis), but they are frequently present in our daily intake of grains. 26
- DON effects on the body 27:
- Enhances T-2 cytokine expression in mice
- Increase expression IL-4, 6,10 and T-2 Toxins
- Suppresses TH1 immune and IFN-gamma production in murine (mice) models
- Potentiates action of IL-1, TNF alpha, and LPS
- Stimulates production of Peyer’s patches (lymph tissue in gut) via MAP kinases (excessive growth can be pathogenic)
- Proinflammatory cytokine production
- COX-2 Expression
- Diarrhea is often associated with DON and this mycotoxin should be considered in the differential of anyone presenting with IBD or diarrhea of unexplained origin. 28
- DON effects on the body 27:
- Binding of Zearalenone and its derivative a-zearalenol. 29 This is a mycotoxin, is a potent estrogenic metabolite produced by some Fusarium and Gibberella species, Zearalenone is the primary toxin, causing infertility, abortion or other breeding problems, especially in swine. Zearalenone is heat-stable and is found worldwide in a number of cereal crops, such as maize, barley, oats, wheat, rice, and sorghum 30
- Binding of Ochratoxin A. 31 (See Earlier comment about this toxin)
- Treatment of acute infectious diarrhea in children 32
- Adjuvant therapy for H. pylori eradication. 33
- Alleviates some symptoms of irritable bowel syndrome 34
BIFIDOBACTERIUM BIFIDUM
- Summary: predominant anaerobic flora in the intestinal tract of breast-fed newborns. Binds to human intestinal mucus. Competes with pathogens for adhesion to the epithelial cells. Increases mucin expression in the jejunum and ileum. Beneficial modulation of the immune system. Strongly down regulates inflammatory responses.
- Bifidobacteria have also been seen to help in cancer prevention and therapy. 35
- Alleviate Chronic constipation. 36
- Alleviates allergy symptoms. 37 38
- Resides in the colon where it enhances nutrient absorption; particularly B-vitamin absorption. Bifidobacterium bifidum also optimizes digestion, maintains a healthy balance in the colon, and prevents histamine production. Histamine is the chemical that triggers allergic reactions.
BIFIDOBACTERIUM INFANTIS
- Treatment of acute infectious diarrhea in children. 39
- Alleviates some symptoms of irritable bowel syndrome. 40
- Antibacterial effect against pathogens such as Clostridia, Shigella, and Salmonella which may show benefit in the protection against gastroenteritis 41
LACTOBACILLUS ACIDOPHILUS
- Binding of Ochratoxin A. 42 (See Earlier comment about this toxin)
- When Lactobacillus acidophilus digests food, lactic acid and hydrogen peroxide are created. These substances kill parasites and prevent more from growing. 43
- Produces natural factors that prevent the overgrowth of the yeast. 44
- Threefold decrease in the incidence of vaginal yeast infections and a reduction in the frequency of Candida colonization in the vagina. 45
- Could be used as an adjuvant treatment during anticancer chemotherapy. 46
- Treatment of acute infectious diarrhea in children. 47
LACTOBACILLUS CASEI
- Alleviates Chronic Constipation. 48
- Advantageous effects include reducing lactose intolerance, alleviating constipation, and even modulation of the immune system. 49
- Reduced the frequency of upper respiratory tract infections in training athletes during the winter. 50
- Helps with Antibiotic associated diarrhea. 51
- Could be used as an adjuvant treatment during anticancer chemotherapy. 52
- Prevention of C. difficile diarrhea in adults. 53
- Adjuvant therapy for H. pylori eradication. 54
- Live L. casei can counteract the pro-inflammatory effects of E. coli on [Crohn’s disease] 55
LACTOBACILLUS SALIVARIUS
- For unprecedented oral health. In fact, this unique strain has been proven through research to dramatically decrease the level of plaque forming bacteria in the mouth while naturally freshening breath and reducing gum sensitivity. 56
- Strong inhibitor of H. Pylori. 57
STREPTOCOCCUS THERMOPHILES
- Significantly reduces the frequency of colic. 58
- Resistant to stomach acid and bile. 59
- Produces lactase thus helps those with lactose intolerance. 60
- Lactase(also known as β-galactosidase ). This enzyme breaks down lactose into β-galactose and glucose.
- Modulates the immune system and has antimicrobial effects. 61
LACTOBACILLUS PARACASEI
- Alleviate obesity and fatty liver
- Decrease fat storage
- Treatment of bacterial vaginosis
- May reduce inflammation and death of intestinal tissue
- Effective in reducing abdominal bloating and pain diverticular patients when combined with a high fiber diet
- May improve immune function
- Alleviation of constipation
- May reduce fatigue related to Chronic Fatigue Syndrome
- May increase antioxidant levels
- Shown to alleviate obesity, insulin resistance and/or hepatic steatosis (Fatty Liver) 62
- (Has been shown to decrease fat storage) Angiopoietin-like 4 (ANGPTL4) is a circulating lipoprotein lipase (LPL) inhibitor that controls triglyceride deposition into adipocytes and has been reported to be regulated by gut microbes.63
- The treatment of bacterial vaginosis is successful. 64
- Results suggest that probiotic supplementation in infants with stage 2 necrotizing enterocolitis may be beneficial in reducing progression of the condition. 65
- In association with a high-fibre diet, is effective in reducing abdominal bloating and prolonged abdominal pain in symptomatic uncomplicated diverticular disease, and could thus be a promising option in the treatment of these patients. 66
- May be an effective means to improve immune function by augmenting systemic and mucosal immune responses to challenge. 67
- Data suggests alleviation of constipation. 68
- Some individuals with Chronic Fatigue Syndrome respond with less fatigue, less bodily symptoms and better neurocognitive functions. 69
- “Athletes and all those exposed to oxidative stress may benefit from the ability of these probiotics to increase antioxidant levels and neutralize the effects of reactive oxygen species.”. 70
LACTOBACILLUS FERMENTUM
- May reduce the development of obesity
- Could be helpful in maintaining remission and preventing relapse of Ulcerative Colitis
- May inhibit the growth of Candida albicans and yeast infections
- May be effective in reducing the recurrence of UTIs
- Able to reduce cholesterol by absorption of cholesterol or causing the body to consume more cholesterol
- Antimicrobial and Antioxidant properties
- Augments healthy aging and resists infections
- “Consumption of Lactobacillus amylovorus or Lactobacillus fermentum bacteria as probiotics may assist in reducing the development of obesity, since these bacteria may confer modifications to energy handling within the host. This creates a microbiome which favors fat oxidation over fat storage, by populating the gut with protective bacteria and preventing the proliferation of pathogenic bacteria, which may ultimately lead to a reduction in body adiposity and transformation of body composition.” Bentley note: While all groups lost significant amounts of body fat, there was a trend toward more fat loss when the probiotics were happily colonizing the intestine… about 2 to 3 pounds vs. about 1 pound with the plain yogurt. 71
- Could be helpful in maintaining remission and preventing relapse of Ulcerative Colitis. 72
- May inhibite the growth of Candida albicans and prevent yeast infections. 73
- May be effective in helping reduce the likelihood of recurrence of UTIs in women. 74
- Inhibit H. pylori (can cause ulcers).
- Increase T-cell activity as needed.
- Has been found to survive stomach acid and bile. 75
- Ability to reduce cholesterol levels, by the absorption of cholesterol, by the incorporation of cholesterol in the host body into its cell membrane or walls. A third mechanism is by causing the body to consume more cholesterol. 76
- Antimicrobial and antioxidative properties. 77
- Alleviates immunosenescence (multi-faceted decline in the function of the immune system during progressive aging), resists infections, and improves anti-oxidant capacity, thereby augmenting healthy aging
LACTOBACILLUS GRASSERI
- May reduce weight gain and adipose tissue
- Associated with weight loss
- May improve diabetic symptoms
- Prevents body weight gain and fat accumulation
- Shown to reduce abdominal adipose tissue
- Analysis of the eligible articles pointed out that Lactobacillus gasseri, Lactobacillus rhamnosus, and the combination of L. rhamnosus and Bifidobacterium lactis may reduce adiposity, body weight, and weight gain. This suggests that these microbial strains can be applied in the treatment of obesity. Furthermore, short chain fatty acid production and low grade inflammation were found as the underlying mechanisms of action that influence metabolism and affect body weight. 78
- Lactobacillus plantarum was associated with weight loss in animals and Lactobacillus gasseri was associated with weight loss both in obese humans and in animals.associated with weight loss both in obese humans and in animals. 79
- Lactobacillus gasseri BNR17 has a suppressing effect on blood glucose levels and improved diabetic symptoms in db/db mice. 80
- Significantly prevented body weight gain, fat accumulation and pro-inflammatory gene expression in the adipose tissue. Relatively lower triglyceride levels and reduced expression of lipogenic genes were also observed in the liver. It is suggested that improvement in the inflammatory state of the adipose tissue might be a possible mechanism underlying the anti-obesity effect. 81
- These findings demonstrate that consumption of LG at doses as low as the order of 10(8) cfu/d exhibited a significant lowering effect on abdominal adiposity, and suggest that constant consumption might be needed to maintain the effect. 82
- Showed lowering effects on abdominal adiposity, body weight and other measures, suggesting its beneficial influence on metabolic disorders. 83
- L. gasseri BNR17 also reduced the levels of leptin and insulin in serum. These results suggest that the anti-obesity actions of L. gasseri BNR17 can be attributed to elevated expression of fatty acid oxidation-related genes and reduced levels of leptin. Additionally, data suggested the anti-diabetes activity of L. gasseri BNR17 may be to due elevated GLUT4 and reduced insulin levels. 84
RESEARCH FOOTNOTES
- Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog. 2012 Aug;53(2):100-8. Epub 2012 May 24.
- B. Klarin et al., “Adhesion of the Probiotic Bacterium Lactobacillus plantarum 299v onto the Gut Mucosa in Critically Ill Patients: A Randomized Open Trial,” Crit. Care 9 (3), R285–293 (2005).
P. Mangell et al., “Lactobacillus plantarum 299v Inhibits Escherichia coli-Induced Intestinal Permeability,” Dig. Dis. Sci. 47 (3), 511–516 (2002). - 9. D.R. Mack et al. , “Probiotics Inhibit Enteropathogenic E. Coli Adherence in vitro by Inducing Intestinal Mucin Gene Expression,” Am. J. Physiol. 276, G941–G950 (1999).
- J. Levy, “The Effects of Antibiotic Use on Gastrointestinal Function,” Am. J. Gastroenterol. 95, S8–S10 (2000).
M. Percival, “Choosing A Probiotic Supplement,” Clin. Nutr. Insights 6, 1–4 (1997).
Y. Aiba et al., “Lactic Acid-Mediated Suppression of Helicobacter pylori by the Oral Administration of Lactobacillus salivarius as a Probiotic in a Gnotobiotic Murine Model,” Am. J. Gastroenterol. 93, 2097–2101 (1998).
P. Gionchetti et al., “Oral Bacteriotherapy as Maintenance Treatment in Patients with Chronic Pouchitis: A Double-Blind, Placebo-Controlled Trial,” Gastroenterol. 119, 305–309 (2000). - J.A. Drisko, C.K. Giles and B.J. Bischoff, “Probiotics in Health Maintenance and Disease Prevention,” Altern. Med. Rev. 8 (2), 143–155 (2003).
- S. Michail and F. Abernathy, “Lactobacillus plantarum Reduces the in vitro Secretory Response of Intestinal Epithelial Cells to Enteropathogenic Escherichia coli Infection,” J. Pediatr. Gastroenterol. Nutr. 35 (3), 350–355 (2002).
- Dalie D.K.D., Deschamps A.M., Richard-Forget F. Lactic acid bacteria – Potential for control of mould growth and mycotoxins: A review (2010) Food Control, 21 (4), pp. 370-380.
- Niku-Paavola, M. L, Laitila, A, Mattila-Sandholm, T., & Haikara, A (1999). New Types of anitmicrobial compounds produced by Lactobacillus plantarum. Journal of Applied Microbiology, 86, 29-35.
- Lavermicocca, P., Valerio, F., & Visconti, A. (2003). Antifungal activity of phenyllactic acidagianst molds isolated from bakery products. Applied and Environmental Microbiology, 69, 634-640.
- Strom, K., Schnurer, J., & Melin, P. (2005) Co-cultivation of antifungal Lactobacillus plantarum MiLAB 393 and Aspergillus nidulans, evaluation of effects on fungal growth and protein expression. FEMS Microbiology Letters, 246 119-124.
Strom, K. Sjogren, J., Broberg, A & Schnurer, J. (2002). Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo (L-Phe-Pro) and cyclo (L-Phe-trans-4-OH-L-Pro) and phenyllactic acid. Applied and Environmental Microbiology, 68, 4322-4327. - Piotrowska, M., & Zakowska, Z. (2005). The limitation of ochratoxin A by lactic acid bacteria strains. Polish Journal of Microbiology, 54, 279–286.
- Al-Anati L, Petzinger E (2006). “Immunotoxic activity of ochratoxin A”. J. Vet. Pharmacol. Ther. 29 (2): 79–90.
- Pfohl-Leszkowicz A, Manderville RA (2007). “Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans”. Mol Nutr Food Res 51 (1): 61–99.
- Blesa J, et al. (2006). “Factors affecting the presence of ochratoxin A in wines”. Critical reviews in food science and nutrition 46 (6): 473–8.
- O’Brien E, Dietrich DR (2005). “Ochratoxin A: the continuing enigma”. Crit. Rev. Toxicol. 35 (1): 33–60.
- Palma N, et al. (2007). “Ochratoxin A-Induced Mutagenesis in Mammalian Cells Is Consistent with the Production of Oxidative Stress”. Chemical Research in Toxicology 20 (7): 1031–1037.
- Belmadani A., et al (1999). “Selective toxicity of ochratoxin A in primary cultures from different brain regions”. Arch Toxicol 73 (2): 108–114.
- Sava V., et al. (2006.). “Acute neurotoxic effects of the fungal metabolite ochratoxin A”. Neurotoxicology 27 (1): 82–92.
- Kunio Doi, Koji Uetsuka (2011). “Ochratoxin A induces apoptosis in neuronal cells”. International Journal of Molecular Sciences 12 : 5213–5327.
- Sanchez M, Darimont C, Drapeau V, et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br J Nutr. 2013:1-13. PMID: 24299712.
- Dalie D.K.D., Deschamps A.M., Richard-Forget F. Lactic acid bacteria – Potential for control of mould growth and mycotoxins: A review (2010) Food Control, 21 (4) , pp. 370-380.
- Haskard, C., El-Nezami, G. S., Kankaanpaa, P. E., Salminen, S., & Ahokas, J.T. (1998) Sequestration of Aflatoxin B1 by probiotic strains: Binding capacity and localization. Revue de Medecine Veterinaire, 149,571.
- Advancing Medicine with Food and Nutrients 2 nd Ed. Pg 839-40
- El-Nezami, H. S., Chrevatidis, A., Auriola, S., Salminen, S., & Mykkänen, H. (2002c). Removal of common Fusarium toxins in vitro by strains of Lactobacillus and Propionibacterium. Food Additives and Contaminants, 19, 680–686.
- International Journal of Molecular Science, 2009. 10(1): p. 1-17
- Advancing Medicine with Food and Nutrients 2 nd Ed. Pg 830
- Medical Mycology 2010. 56: p. 282-294, Toxicology and Applied Pharmacology, 2008. 228(1): p. 84-92
- Advancing Medicine with Food and Nutrients 2 nd Ed. Pg 834
- El-Nezami, H. S., Polychronaki, N., Salminen, S., & Mykkänen, H. (2002a). Binding rather metabolism may explain the interaction of two food-grade Lactobacillus strains with zearalenone and its derivative a-zearalenol. Applied and Environmental Microbiology, 68, 3545–3549.
- Kuiper-Goodman, T.; Scott, P. M.; Watanabe, H. (1987). “Risk Assessment of the Mycotoxin Zearalenone”. Regulatory Toxicology and Pharmacology 7 (3): 253–306.
- Piotrowska, M., & Zakowska, Z. (2005). The limitation of ochratoxin A by lactic acid bacteria strains. Polish Journal of Microbiology, 54, 279–286.
- Allen SJ, Okoko B, Martinez E, Gregorio G, Dans LF. Probiotics for treating infectious diarrhoea. Cochrane Database Syst Rev 2004;(2):CD003048.
- Tong JL, Ran ZH, Shen J, Zhang CX, Xiao SD. Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther 2007;25:155–68.
- Gawronska A, Dziechciarz P, Horvath A, Szajewska H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther 2007; 25: 177–84.
- Yoshinori HAMAJI, Minoru FUJIMORI, Takayuki SASAKI, Hitomi MATSUHASHI, Keiichi MATSUI-SEKI, Yuko SHIMATANI-SHIBATA, Yasunobu KANO, Jun AMANO and Shun’ichiro TANIGUCHI, “Strong Enhancement of Recombinant Cytosine Deaminase Activity in Bifidobacterium longum for Tumor-Targeting Enzyme/Prodrug Therapy”, Biosci. Biotechnol. Biochem., Vol. 71, 874-883 (2007).
- Amenta, Michele et al. “Diet and chronic constipation. Benefits of oral supplementation with symbiotic zir fos (Bifidobacterium longum W11 + FOS Actilight)”. Acta Biomed. Published December 2006.
- Xiao,JZ et al. “Clinical Efficacy of Probiotic Bifidobacterium longum for the Treatment of Symptoms of Japanese Cedar Pollen Allergy in Subjects Evaluated in an Environmental Exposure Unit”. Allergology International. Published March 2007.
- Ohno Hiroshi, Tsunemine Satoru, Isa Yasuhiro, Shimakawa Masaki, Yamamuru Hideki. Oral Administration Bifidobacterium bifidum G9-1 Suppresses Total and Antigen SpecificImmunoglobulin E Production in Mice. Biological and Pharmaceutical Bulletin 28(8)pp.1462-1466 20050801
- Allen SJ, Okoko B, Martinez E, Gregorio G, Dans LF. Probiotics for treating infectious diarrhoea. Cochrane Database Syst Rev 2004;(2):CD003048.
- O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 2005; 128:541–51.
- Gibson GR, Wang X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol. 1994 Oct;77(4):412-20.
- Piotrowska, M., & Zakowska, Z. (2005). The limitation of ochratoxin A by lactic acid bacteria strains. Polish Journal of Microbiology, 54, 279–286.
- “Lactobacillus Acidophilus.” University of Maryland Medical Center. http://www.umm.edu/altmed/articles/lactobacillus-acidophilus-000310.htm.
- Fitzsimmons N, Berry DR. Inhibition of Candida albicans by Lactobacillus acidophilus: evidence for the involvement of a peroxidase system. Microbios 1994;80:125–33.
- Hilton E, Isenberg HD, Alperstein P, et al. Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann Intern Med 1992;116:353–7.
- Millette, M. et al. 2010. Probiotic Lactobacillus acidophilus and L. casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutrition and Cancer. 62(3):371-8.
- Allen SJ, Okoko B, Martinez E, Gregorio G, Dans LF. Probiotics for treating infectious diarrhoea. Cochrane Database Syst Rev 2004;(2):CD003048.
- Koebnick C, Wagner I, Leitzmann P, Stern U, Zunft HJF. Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can J Gastroenterol 2003;17:655-9.
- Lactobacillus Casei, published by Citizendium, The Citizens’ Compendium, 2009.
- Gleeson M, Bishop NC, Oliveira M, Tauler P. Daily probiotic’s (Lactobacillus casei Shirota)reduction of infection incidence in athletes. Int J Sport Nutr Exerc Metab 2011;21:55–64.
- Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010 Jul;105(7):1636-41.
- Millette, M. et al. 2010. Probiotic Lactobacillus acidophilus and L. casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutrition and Cancer. 62(3):371-8.
- Plummer S, Weaver MA, Harris JC, et al. Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of Clostridium difficile diarrhoea. Int Microbiol 2004;7:59–62.
- Sýkora J, Valecková K, Amlerová J, et al. Effects of a specially designed fermented milk product containing probiotic Lactobacillus casei DN-114 001 and the eradication of H. pylori in children: a prospective randomized double-blind study. J Clin Gastroenterol 2005;39:692–8.
- Lactobacillus Casei Downregulates Commensals’ Inflammatory Signals in Crohn’s Disease Mucosa, published in inflammatory Bowel Diseases, Wiley InterScience, 2008.
- Mayanagi G, Kimura M, Nakaya S, Hirata H, Sakamoto M, Benno Y, Shimauchi H. Probiotic effects of orally administered Lactobacillus salivarius WB21-containing tablets on periodontopathic bacteria: a double-blinded, placebo-controlled, randomized clinical trial; J Clin Periodontol. 2009 Jun;36(6):506-13. Epub 2009 Apr 22.
Iwamoto T, Suzuki N, Tanabe K, Takeshita T, Hirofuji T. Effects of probiotic Lactobacillus salivarius WB21 on halitosis and oral health: an open-label pilot trial; Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010 Aug;110(2):201-8. - Ryan KA, Daly P, Li Y, Hooton C, O’Toole PW. Strain-specific inhibition of Helicobacter pylori by Lactobacillus salivarius and other lactobacilli. J Antimicrob Chemother. 2008 Apr;61(4):831-4.
- Saavedra JM, Abi-Hanna A, Moore N, Yolken RH. Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. Am J Clin Nutr 2004;79:261–7.
- Hatice Boke, Belma Aslim, and Gulcin Alp, The Role of Resistance to bile salts and acid tolerance of Exopolysaccharideis (EPSS) produced by yogurt starter bacteria. Arch. Biol. Sci., Belgrade, 62 (2), 323-328, 2010
- Drouault, S ; Anba, J ; Corthier, G, Streptococcus thermophilus is able to produce a beta-galactosidase active during its transit in the digestive tract of germ-free mice. APPLIED AND ENVIRONMENTAL MICROBIOLOGY Volume: 68 Issue: 2 Pages: 938-941
- Sheil B, Shanahan F, O’Mahony L. Probiotic effects on inflammatory bowel disease. J Nutr. 2007 Mar;137(3 Suppl 2):819S-24S.
- Wang J, Tang H, Zhang C, et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. The ISME Journal . 2015;9(1):1-15. doi:10.1038/ismej.2014.99.
- Aronsson L, Huang Y, Parini P, Korach-André M, Håkansson J, Gustafsson J-Å, et al. (2010) Decreased Fat Storage by Lactobacillus Paracasei Is Associated with Increased Levels of Angiopoietin-Like 4 Protein (ANGPTL4). PLoS ONE 5(9): e13087. doi:10.1371/journal.pone.0013087
- Delia A, Morgante G, Rago G, Musacchio MC, Petraglia F, De Leo V. Effectiveness of oral administration of Lactobacillus paracasei subsp. paracasei F19 in association with vaginal suppositories of Lactobacillus acidofilus in the treatment of vaginosis and in the prevention of recurrent vaginitis. Minerva Ginecol. 2006. 58:227–231
- Zampieri N, Pietrobelli A, Biban P, et al. Lactobacillus paracasei subsp. paracasei F19 in Bell’s stage 2 of necrotizing enterocolitis. [Journal Article, Randomized Controlled Trial] Minerva Pediatr 2013 Aug; 65(4):353-60.
- Annibale B, Maconi G, et al, “Efficacy of Lactobacillus paracasei sub. paracasei F19 on abdominal symptoms in patients with symptomatic uncomplicated diverticular disease: a pilot study,” Minerva Gastroenterol Dietol, 2011 March; 57(1): 13-22.
- Rizzardini, G., Eskesen, D., Calder, P.C., Capetti, A., Jespersen, L., Clerici, M. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12(R) and Lactobacillus paracasei ssp. paracasei, L. casei 431(R) in an influenza vaccination model: a randomised, double-blind, placebo-controlled study. Br J Nutr. 2012;107:876–884.
- Valerio F, Russo F de Candia S, Riezzo G, Orlando A, Lonigro SL, Lavermicocca P.Effects of probiotic Lactobacillus paracasei-enriched artichokes on constipated patients: a pilot study J Clin Gastroenterol. 2010 Sep;44 Suppl 1:S49-53. doi: 10.1097/MCG.0b013e3181d2dca4.
- Sullivan Å , Nord CE, Eveng å rd B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutrition Journal . 2009;8:4. doi:10.1186/1475-2891-8-4.
- Martarelli D , Verdenelli MC , Scuri S , Cocchioni M , Silvi S , Cecchini C , Pompei P . Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr Microbiol. 2011 Jun;62(6):1689-96.
- Omar JM, Chan YM, Jones ML, Prakash S, Jones PJH. Lactobacillus fermentum and Lactobacillus amylovorus as probiotics alter body adiposity and gut microflora in healthy persons. J Funct Foods . 2013;5(1):116-123.
- Hegazy SK, El-Bedewy MM. Effect of probiotics on pro-inflammatory cytokines and NF-kappaB activation in ulcerative colitis. World J Gastroenterol. 2010 Sep 7;16(33):4145-51.
- Cadieux P, Burton J, Reid G, et al, “ Lactobacillus Strains and Vaginal Ecology,” JAMA, April 17, 2002;287(15):1940-1941.
- Falagas ME, Betsi GI, et al, “Probiotics for prevention of recurrent urinary tract infections in women : a review of the evidence from microbiological and clinical studies,” Drugs, 2006; 66(9): 1253-1261.
- Pan, Dao D., Xia Q. Zeng, and Tian Yan. “Characterization of Lactobacillus fermentum SM-7 isolated from koumiss, a potential probiotic bacterium with cholesterol-lowering effects.” Journal of the Science of Food and Agriculture 91, no. 3 (2011): 512-518.
- Pan, Dao D., Xia Q. Zeng, and Tian Yan. “Characterization of Lactobacillus fermentum SM-7 isolated from koumiss, a potential probiotic bacterium with cholesterol-lowering effects.” Journal of the Science of Food and Agriculture 91, no. 3 (2011): 512-518.
- Mikelsaar, Marika, and Mihkel Zilmer. “Lactobacillus fermentum ME-3 An antimicrobial and antioxidative probiotic.” Microbial Ecology in Health and Disease 21, no. 1 (2009): 1-27.
- Mekkes MC, Weenen TC, Brummer RJ, et al. (2013) The development of probiotic treatment in obesity: a review. Benef Microbes 5, 19–28.
- Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog. 2012;53: 100–8 doi: 10.1016/j.micpath.2012.05.007 [ PubMed ]
- Yun SI, Park HO, Kang JH. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol. 2009;107:1681–1686.
- Miyoshi M, Ogawa A, Higurashi S, Kadooka Y. Anti-obesity effect of Lactobacillus gasseri SBT2055 accompanied by inhibition of pro-inflammatory gene expression in the visceral adipose tissue in diet-induced obese mice. Eur J Nutr. 2014;53:599–606. doi: 10.1007/s00394-013-0568-9.
- Kadooka Y, Sato M, Ogawa A, et al. (2013) Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br J Nutr 110, 1696–1703.
- Kadooka Y, Sato M, Imaizumi K, et al. (2010) Regulation of abdominal adiposity by probiotics ( Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 64, 636–643.
- Kang J-H, Yun S-I, Park M-H, Park J-H, Jeong S-Y, Park H-O. Anti-Obesity Effect of Lactobacillus gasseri BNR17 in High-Sucrose Diet-Induced Obese Mice. Maedler K, ed. PLoS ONE . 2013;8(1):e54617. doi:10.1371/journal.pone.0054617.
Try it now
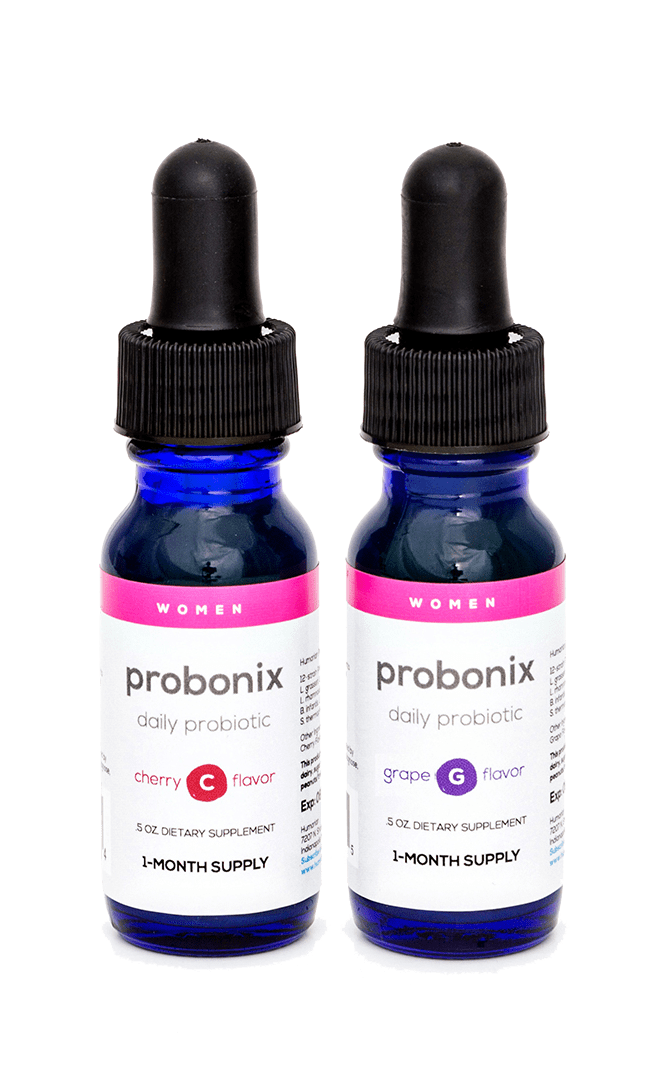
Enter code TRYITNOW20
for 20% Off Your First Order
Our liquid probiotic uses an exclusive formula containing 12 of the most effective probiotic strains to help with gut health, yeast infections, and urinary tract infections. Could help if you have issues like:
- Urinary tract infections
- Vaginal health
- Yeast infections
- Irritable Bowel Syndrome (IBS)
- Bloating
- Gas
- And more!
Probonix is an organic, non-GMO, and all natural supplement free from gluten, dairy, sugar, soy, eggs, fish/shellfish, and peanuts/tree nuts.
Taking Probonix is simple. Just put eight drops on your tongue or in your favorite unheated drink once a day.
Description: 0.5 oz bottle, 30 Day Supply
Suggested Dose: 8 drops daily for women
